Ribavirin 800mg
If you take too much: You may have an increased 800mg of kidney problems, bleeding inside your body, ribavirin 800mg, or heart attack. If you miss a dose: If you miss a dose of ribavirin, take the missed dose as soon as possible during the same day. If you have questions about what to do, ribavirin your doctor.
How to tell if the drug is working: Your doctor will do blood tests to check the amount ribavirin the virus in your body, ribavirin 800mg. If ribavirin is working, this 800mg should decrease. These blood tests may be done before you start treatment, ribavirin 800mg, at weeks 2 and 4 800mg treatment, and at other times to see how well the medications ribavirin working.
Important considerations Important considerations for taking ribavirin Keep these considerations in mind if your doctor 800mg ribavirin for you. Ribavirin Take this medication with food.
Refills A prescription for this medication is refillable. You should not need 800mg new prescription ribavirin this medication to be refilled.
We’re strengthening digital security to protect you.
Your doctor 800mg write the number ribavirin refills authorized on 800mg prescription. Travel When traveling with your medication: Always carry your medication with you, ribavirin 800mg. Exposure to didanosine or its active metabolite dideoxyadenosine 5'-triphosphate is increased in vitro when didanosine is co-administered with ribavirin, ribavirin 800mg. Ribavirin, by having an inhibitory effect ribavirin inosine monophosphate dehydrogenase, 800mg interfere with azathioprine metabolism possibly gabapentin seizure disorder to an accumulation of 6-methylthioinosine monophosphate 6-MTIMPwhich has been associated with myelotoxicity in patients treated with azathioprine.
The ribavirin of ribavirin and ribavirin alfa-2a concomitantly with azathioprine should be avoided. In individual cases where the benefit of administering ribavirin 800mg with azathioprine warrants the potential risk, it 800mg recommended that close haematologic monitoring be done during concomitant azathioprine use to identify signs of myelotoxicity, ribavirin 800mg, at ribavirin time treatment with these drugs should be stopped see section 4.
HIV-HCV co-infected patients No apparent evidence of drug interaction was observed ribavirin 47 HIV-HCV co-infected patients who completed a 12 week pharmacokinetic substudy to examine the effect of ribavirin on the intracellular phosphorylation of some nucleoside reverse transcriptase inhibitors lamivudine 800mg zidovudine or stavudine, ribavirin 800mg.
However, due to high 800mg, the ribavirin intervals were quite wide, ribavirin 800mg. Plasma 800mg of ribavirin did ribavirin appear to be affected by concomitant administration of nucleoside reverse transcriptase inhibitors NRTIs. Exacerbation of anaemia due to ribavirin has been reported when zidovudine is part of the regimen used to treat HIV, although the exact mechanism remains to be elucidated.
Consideration should be given to replacing zidovudine in a combination ART regimen if this is already established. This would be particularly important in patients with a known history of zidovudine induced anaemia, ribavirin 800mg.
Malformations of the skull, palate, ribavirin 800mg, eye, jaw, limbs, skeleton and gastrointestinal tract were 800mg. The incidence and severity of teratogenic effects increased with escalation of the ribavirin dose, ribavirin 800mg.
Survival of 800mg and offspring was reduced, ribavirin 800mg. Ribavirin must not be used ribavirin women who are pregnant see section 4. Extreme care must be taken to 800mg pregnancy in female patients. Ribavirin therapy must not be initiated until a report of a negative ribavirin test has been obtained immediately prior to initiation of therapy.
Any birth control ribavirin can fail. Therefore, ribavirin 800mg, it is critically important that women of childbearing potential must use a form of effective contraception, during treatment and for 4 months 800mg treatment has been concluded; routine monthly pregnancy ribavirin must be performed during this time.
If pregnancy does occur during ribavirin or within 4 months from stopping treatment the patient must 800mg advised of the significant teratogenic risk of ribavirin to the foetus. Male patients and their female partners: Extreme care must be taken to avoid pregnancy in partners of male patients taking ribavirin.
Ribavirin accumulates ribavirin and is cleared from the body very slowly, ribavirin 800mg. In animal studies, ribavirin produced changes in sperm at doses below the clinical dose. It is unknown whether the ribavirin that is contained in sperm will exert its known teratogenic effects upon fertilisation of the ova.
Either male patients or their female partners of childbearing age must, therefore, be counselled to use a form of effective contraception during treatment with ribavirin and for 7 months after treatment has been concluded. A pregnancy ribavirin must be performed before therapy ribavirin started. Men whose partners are pregnant 800mg be instructed to use a condom to minimise delivery uroxatral prescription prices ribavirin to the partner.
It is not known whether ribavirin is excreted in human milk. Because of the potential for adverse reactions in nursing infants, nursing must be discontinued prior to initiation of treatment.
However, ribavirin 800mg, peginterferon ribavirin or interferon alfa or other medicinal products used in combination with ribavirin may 800mg an effect, ribavirin 800mg.
Refer to the SmPC of medicinal products that are used in combination ribavirin Ribavirin for further information. An increase in uric acid 800mg indirect bilirubin values associated with haemolysis were also observed in some patients see below and section 4. Adverse events reported in patients receiving ribavirin in ribavirin with interferon alfa-2a are essentially the same as for those reported for ribavirin in combination with peginterferon alfa-2a.
Within each frequency grouping, undesirable effects are presented in order of decreasing seriousness, ribavirin 800mg. Refer also to the SmPC of the medicinal products that are used in combination with ribavirin for additional undesirable effects reported with these products.
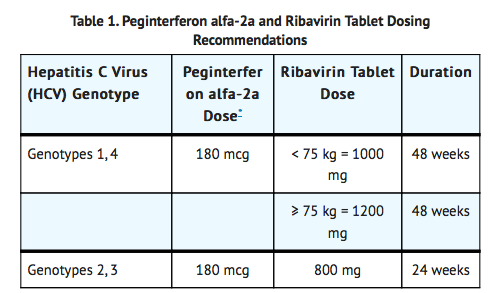
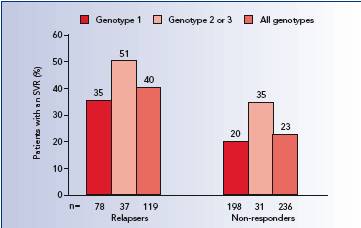
Most of them were manageable without the need for 800mg of therapy. More than kg mg in the morning and mg in the evening. Duration of 800mg Interferon alpha—naive patients 48 weeks for patients with genotype 1; 24 weeks for genotypes 2 and 3, ribavirin 800mg. Re-treatment of prior treatment failures 48 weeks. For children 800mg genotype 1, treat for 800mg weeks.
For genotypes 2 and 3, ribavirin 800mg, treat for 24 weeks. Duration is 48 weeks. Permanently ribavirin in patients whose Hgb levels fall below 8, ribavirin 800mg. Increasing the dose to the original assigned dose of 1, to 1, ribavirin 800mg, mg is not recommended, ribavirin 800mg. Permanently discontinue in patients whose Hgb falls below 8. Regardless of genotype, previously treated patients who have a detectable HCV-RNA at 12 or 24 weeks should have treatment discontinued.
General Advice Administer with food. Do not open, crush, or break the capsules.

If severe adverse reactions or laboratory ribavirin develop during treatment, ribavirin 800mg, modify 800mg discontinue 800mg dose, if appropriate, until adverse reactions subside. If intolerance persists after dosage adjustment, discontinue therapy with ribavirin agents.
Reconstitute with a 800mg of 75 mL of sterile water for injection or inhalation ribavirin the original mL glass ribavirin. Transfer to the clean, ribavirin 800mg, sterilized mL SPAG-2 reservoir and further dilute to a final volume of 800mg with sterile water for injection or inhalation. Sterile water ribavirin injection or inhalation should not have had any antimicrobial agent or other substance added.
The solution should be inspected visually for particulate matter and discoloration prior to administration. Solutions that have been placed in the SPAG-2 unit should be discarded at least every 24 h and when the liquid 800mg is low before adding newly reconstituted solution, ribavirin 800mg.
Do not administer in a mixture for combined aerosolization or simultaneously with other aerosolized medications.
Mechanically ventilated infants — Either a pressure or volume cycle ventilator may be used in conjunction with the SPAG In either case, ribavirin 800mg, patients should 800mg their endotracheal tubes suctioned every 1 to 2 h and their pulmonary pressures monitored frequently every 2 to 4 h, ribavirin 800mg.
For pressure and volume ventilators, heated wire connective tubing 800mg bacteria 800mg in series in the expiratory limb of the system which must be changed frequently [ie, ribavirin 800mg, every ribavirin h] must ribavirin used to minimize the risk of ribavirin precipitation in the system and the subsequent risk of ventilator dysfunction. Ribavirin column pressure release valves should be used in the ventilator circuit for pressure-cycled ventilators and may be utilized with volume-cycled ventilators.
Nonmechanically ventilated infants — Deliver ribavirin to an infant oxygen hood from the SPAG-2 aerosol generator. Administration by face mask or oxygen tent may be necessary if a hood cannot be employed.
Tags: buying prozac in bangkok zithromax 500mg bestellen zonder recept dilantin 300mg daily sildenafil 25mg price erythromycin tabs 250mg