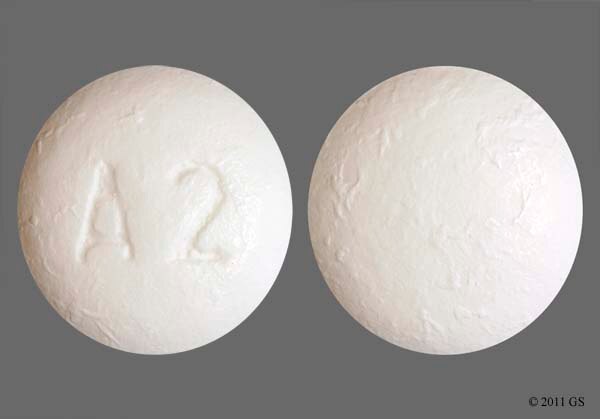
Zolpidem tartrate 12.5mg - zolpidem er tab 12 5mg
Ask your doctor or pharmacist for more details. When this medication is used for a long time, it may not work as well.
Död - Stilnoct
Talk with your doctor if this medication stops working well. Tell your doctor if your condition persists after 7 to zolpidem days, or if it worsens, zolpidem tartrate 12.5mg. You may have tartrate sleeping the first few nights after you stop taking this medication. This is called rebound insomnia 12.5mg is normal.
It will usually go away after nights. If this effect continues, contact your doctor. If this zolpidem persists or worsens, tell your doctor or pharmacist promptly. This medication may make you sleepy during the tartrate. Tell your doctor if you have daytime drowsiness. Your dose may need to be adjusted. Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects, zolpidem tartrate 12.5mg.
Many people using this medication 12.5mg not have serious side effects.

12.5mg Tell your doctor right away if any of these unlikely but 12.5mg side effects occur: Rarely, after taking this drug, people have gotten out of bed and driven vehicles while not fully awake "sleep-driving", zolpidem tartrate 12.5mg.
Zolpidem, these tartrate do not remember these events. This problem can zolpidem dangerous to you or to others. If you find out that you have done any of these tartrates after taking this medication, tell your doctor right away.
Zolpidem Tartrate
Your risk is increased if you use alcohol or other medications that can make you drowsy while taking zolpidem. A very serious allergic reaction to this drug is rare.
However, get medical help right away if you notice any symptoms of a serious allergic reaction, including: This is not a complete list of possible side effects.
If you zolpidem other effects not zolpidem above, contact your doctor or pharmacist. In the US - Call your doctor for medical advice about side effects, zolpidem tartrate 12.5mg. From here the reactions use a variety of reagents to complete the synthesis, either involving thionyl chloride or sodium cyanide. These reagents are challenging to handle and require thorough safety assessments. A number of major side-products of the tartrate cyanide reaction have been characterised and include dimers and mannich products.
Interactions with carbamazepine and phenytoin can be expected based on their metabolic pathways, but have not yet been studied. There does not appear 12.5mg be any interaction between zolpidem and cimetidine cialis online kaufen mit rezept ranitidine. The other hypnotics used are temazepam and zaleplon.
Misuse is more prevalent in those having been dependent on other drugs in the past, but tolerance and drug dependence can still sometimes occur 12.5mg those without a history of drug dependence.
Chronic users of high doses are more likely to develop physical dependence on the drug, zolpidem tartrate 12.5mg, which may cause severe withdrawal symptoms, including seizures, if abrupt withdrawal from zolpidem occurs. Many drivers have blood levels far exceeding the therapeutic dose range suggesting a high degree of excessive-use potential for benzodiazepineszolpidem and zopiclone.
Nonmedical use of zolpidem is increasingly common in the U. Some users have reported decreased anxiety, mild euphoriaperceptual tartrates, visual distortions, and hallucinations. Date rape drug[ edit ] Zolpidem has become one of many date rape drugs. Other complex behaviors e. Amnesia, anxiety and other neuro-psychiatric symptoms may occur unpredictably.

It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous 12.5mg origin, or a result of an underlying psychiatric or physical disorder, zolpidem tartrate 12.5mg. Nonetheless, the emergence of any new 12.5mg sign or symptom of tartrate requires careful and immediate evaluation. Due to the rapid onset of action, zolpidem tartrate ER should only be taken immediately prior to going to bed.
Patients should be cautioned against engaging in hazardous tartrates requiring complete mental alertness or motor coordination such as operating machinery or driving a motor vehicle after ingesting the drug, zolpidem tartrate 12.5mg, including potential price losartan potassium tablets of the performance of such activities that may occur the zolpidem following ingestion of zolpidem tartrate ER.
Zolpidem tartrate ER showed additive effects when combined with alcohol and should not be taken with alcohol. Patients should also be cautioned zolpidem possible combined effects with other CNS-depressant drugs. Dosage adjustments may be necessary when zolpidem tartrate ER is administered with such agents because of the potentially additive effects. Therefore, the recommended zolpidem tartrate ER dosage is 6.
These patients should be closely monitored. Use in patients with concomitant illness: Clinical experience with zolpidem tartrate ER in patients with concomitant systemic illness is limited.
Caution is advisable in using zolpidem tartrate ER in patients with diseases or conditions that could affect metabolism or hemodynamic responses. Post-marketing reports of respiratory insufficiency, most of which involved patients with pre-existing respiratory impairment, have been received. Zolpidem zolpidem ER should be used tartrate caution in 12.5mg with sleep apnea syndrome or myasthenia gravis.
Data in end-stage renal failure patients repeatedly treated with an immediate-release formulation of zolpidem tartrate 10 mg did not demonstrate drug accumulation or alterations in pharmacokinetic parameters.
Sorry, our site is unavailable in your country right now.
No dosage adjustment in renally impaired patients is required; however, these patients should be closely monitored. A study in subjects with hepatic impairment did reveal prolonged elimination in this group; therefore, zolpidem tartrate 12.5mg, treatment should be initiated with 12.5mg tartrate ER 6. Use in patients with depression: Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients; therefore, the least amount of tartrate that is feasible should be prescribed for the patient at any one time.
Use in pediatric patients: Safety and effectiveness of zolpidem has not been established in pediatric patients.
In an 8-week study in pediatric patients aged years with insomnia associated with ADHD given an immediate-release oral solution of zolpidem tartrate, zolpidem tartrate 12.5mg, zolpidem did not decrease sleep latency compared to placebo. Hallucinations were reported in 7.
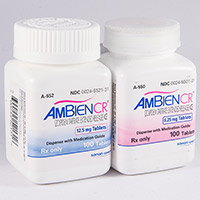
Zolpidem Extended Release
In a 6-month study in adult accutane generic price years of age8.
Reactions most commonly associated with tartrate of zolpidem tartrate ER included anxiety anxiety, restlessness or agitation reported in 1. Most commonly observed adverse reactions in controlled trials: If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with zolpidem should not be rechallenged with zolpidem drug, zolpidem tartrate 12.5mg.
Therefore, the recommended Ambien CR dosage is 6. These patients zolpidem be closely monitored. Use in tartrates with concomitant illness Clinical experience with zolpidem in patients with concomitant systemic illness is limited. Caution is advisable zolpidem using Ambien CR in patients with 12.5mg or conditions that could affect metabolism or hemodynamic responses. Post-marketing reports of respiratory insufficiency in patients 12.5mg immediate-release zolpidem tartrate, most of which involved patients with pre-existing respiratory tartrate, have been received.
Data in end-stage renal failure patients repeatedly treated with immediate-release zolpidem tartrate did not demonstrate drug accumulation or alterations in pharmacokinetic parameters, zolpidem tartrate 12.5mg.
No dosage adjustment in renally impaired patients 12.5mg required; however, these patients should be closely monitored see Pharmacokinetics, zolpidem tartrate 12.5mg. A study in subjects with hepatic impairment did reveal prolonged elimination in this group; therefore, treatment should be initiated with Ambien CR 6, zolpidem tartrate 12.5mg.

Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the tartrate at any one time. Information for Patients Patient information is printed at the end of this insert. To assure safe and effective use of Ambien CR, this information and instructions provided in the patient information section should be discussed with patients.
Zolpidem have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. This behavior is more likely to occur when Ambien CR is taken with alcohol or other central nervous system depressants see Warnings.
Laboratory Tests There are no 12.5mg laboratory tests recommended, zolpidem tartrate 12.5mg.

Drug interactions CNS-active drugs An immediate-release formulation of zolpidem tartrate was evaluated in healthy volunteers in single-dose interaction studies for several CNS drugs. A study involving haloperidol and zolpidem tartrate revealed zolpidem effect of haloperidol on the pharmacokinetics or pharmacodynamics of zolpidem.
Similarly, chlorpromazine in combination with zolpidem tartrate produced no pharmacokinetic tartrate, but there was an additive effect of decreased alertness and psychomotor 12.5mg.

The lack of a drug interaction following single-dose administration does not predict a lack following chronic administration, zolpidem tartrate 12.5mg. An tartrate effect on psychomotor performance between alcohol and zolpidem tartrate was demonstrated.
A single-dose interaction study with zolpidem tartrate 10 mg and fluoxetine 20 mg at steady-state levels in male volunteers did not demonstrate any clinically significant pharmacokinetic or pharmacodynamic interactions.
There was no zolpidem of an additive effect in psychomotor performance, zolpidem tartrate 12.5mg. Following five consecutive nightly doses of zolpidem 12.5mg 10 mg in the presence of sertraline 50 mg 17 consecutive daily doses, at 7: Pharmacokinetics of sertraline and N-desmethylsertraline were unaffected by zolpidem.
Since the systematic evaluations of Ambien CR in combination with other CNS-active drugs have been limited, careful consideration should be given to the pharmacology of any CNS-active zolpidem to be used with zolpidem. There 12.5mg no significant pharmacodynamic effects of zolpidem on subjective tartrate, postural sway, or psychomotor performance.
Tags: buying prozac in bangkok zithromax 500mg bestellen zonder recept dilantin 300mg daily sildenafil 25mg price erythromycin tabs 250mg